depicted in CPK coloring (oxygens highlighted in red, nitrogens in blue and chlorines in green)
and artistically contrasted from its target protein.
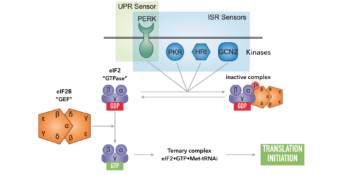
Cells maintain their proteins in a functional and balanced state by regulating protein synthesis, folding, trafficking and degradation. A central regulatory target in this process is the nucleotide exchange factor eIF2B. Under favorable conditions eIF2B acts as a biological catalyst, efficiently unloading GDP from translation initiation factor 2 (eIF2), a GTPase that is required for protein synthesis. Under conditions of stress, such as UPR activation, viral infection, or starvation, a conserved signaling network known as the integrated stress response (ISR) couples stress detection to the phosphorylation of eIF2. Phosphorylated eIF2 in turn inhibits eIF2B and arrests translation to mitigate stress.
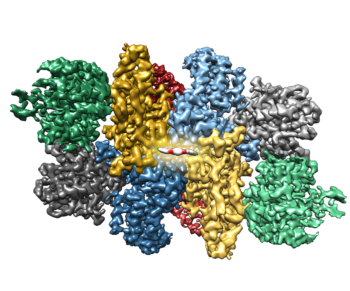
We discovered a small molecule called ISRIB (integrated stress response inhibitor) that restores translation during stress by activating eIF2B. Remarkably, ISRIB enhances cognition and reverses cognitive deficits following brain injury in rodents. These effects highlight the potential of targeting the ISR for therapeutics. Recent work in the lab has determined the structure of eIF2B bound to ISRIB and a mechanism of action for the molecule. Future studies will focus on the critical regulation of this major translational control point.